SUMMARY
A 38-year-old cattleman died as a result of an accidental injection of an animal antibiotic known as Micotil which has no known antidote. On March 8, 2003, the victim was preparing to vaccinate a heifer inside a barn. He was carrying a 12cc plastic disposable syringe in his right hand when a cow that was in an adjacent pen charged him, striking the fence between the two. The victim was knocked to the ground. Either when struck or from the fall, he was injected with an unknown amount of the antibiotic. He immediately began to feel dizzy and nauseous. He was able to return to the “vet room” inside the barn and call his wife who was nearby in the house. An ambulance was called and the victim was rushed to a nearby hospital where he died less than an hour later.
The Nebraska Workforce Development, Department of Labor’s Investigator concluded that to help prevent future similar occurrences:
PROGRAM OBJECTIVE
The goal of the Fatality Assessment and Control Evaluation (FACE) workplace investigation is to prevent future work-related deaths or injuries, by a study of the working environment, the worker, the task the worker was performing, the tools the worker was using, and the role of management in controlling how these factors interact.
This report is generated and distributed solely for the purpose of providing current, relevant education to employers, their employees and the community on methods to prevent occupational fatalities and injuries.
INTRODUCTION
On March 8, 2003 at approximately 7 p.m. a 38-year-old cattleman died within an hour as a result of an accidental injection of the bovine antibiotic Micotil 300®. The Nebraska Department of Labor was notified of the incident on March 9, 2003 through the local news media. The Nebraska FACE investigator met with the victim’s family on June 10th & 12th. Site visits were conducted both days. The local emergency response personnel that responded to the incident site were interviewed on June 12th. A telephone conference was held with the drugs’ manufacturer, ELANCO on June 16th . and with the Federal Drug Administrations’ (FDA) Center for Veterinary Medicine on June 19th.
The victim was a self-employed cattleman/farmer. He was born and raised on a farm and had been raising cattle virtually his entire life. At the time of the incident the farm did not employ any other personnel. The victim was in good physical shape.
INVESTIGATION
On the day of the incident the victim was working alone inside the barn. He had brought a young heifer (young cow that has not previously calved) into the barn and placed her in a squeeze chute (see photo #2). His intent was to inject her with the antibiotic Micotil 300®. He went to the “vet room”, located inside the barn where supplies and medicine were kept (see photo #1). He probably withdrew between 10-12 ccs of the antibiotic (normal dosage requirement for this sized animal) from a 100 ml bottle, into a plastic disposable syringe and left the room with the syringe believed to be carried in his right hand. As he walked down an alleyway towards the squeeze chute (see photo #3), he had to pass by a metal swing gate that was post-attached via hinges, enclosing a fenced-in pen that ran parallel along the east side (right side of victim) of the barn. This pen contained a “horned cow” that was ready to calve at any time. As the victim started to open the gate and walk through to the squeeze chute, the horned cow charged, striking the fence panel and/or the victim with enough force to knock him to the ground (see photo #4). As he tried to regain his balance, he began feeling dizzy. He was able to make it approximately 25 feet back down the alleyway to the “vet room” and use a phone located inside the door to call his wife in the nearby house. She found him extremely dizzy and becoming nauseous. 911 was called along with a neighbor. The local emergency rescue squad (EMTs) responded from a nearby town within a few minutes and immediately transported the victim to a nearby hospital. En route they were met by an ambulance containing paramedics that could provide more care if needed.
| Photo #1. Looking south down walkway towards vet room door on right side. Phone was located directly inside door on right side mounted to the wall. |
| Photo #2. Squeeze chute where heifer was being held. |
| Photo #3. This is the walkway between the “vet room” on the left and cattle holding pens on the right. The squeeze chute that held the heifer is to the left through the opening just beyond the metal gate. |
| Photo #4. Metal gate on the left is hinged on the left side and swung against the pen to allow the heifer, once inoculated, to leave the squeeze chute and exit the barn going through the opening on the left, down the walkway and out a door on the side. The victim was passing between it and the metal pipe panel on the right side when the horned cow charged, knocking him to the ground. The pipe is 1¼ inch diameter and there is 8 inches in between pipes. |
Information concerning medications and procedures administered at the incident site and during ambulance transport were not revealed to the investigator due to doctor/patient confidentiality guidelines of both agencies involved.
The syringe and needle that the victim had been carrying was located at the incident site and was slightly bent, indicating that the victim had probably been injected with some of the antibiotic. This information was relayed to the emergency responders and hospital personnel and they contacted a poison control center. When the ambulance arrived at the hospital, the victim was told by the treating physician that there was no known antidote that could help him. The victim knew his family had arrived at the hospital and wanted to talk with them, but collapsed and died while getting off the emergency room table.
Later that evening the victim’s father returned to the barn to check on the status of the heifer in the squeeze chute. As he passed between the swing gate and pen fence where the incident occurred, the horned cow again charged the fence panel. After releasing the heifer from the squeeze chute he again passed in the opposite direction between the swing gate and pen, causing the cow to charge him a second time. It can be concluded with reasonable certainty that this charging cow was responsible for knocking the victim down.
A request was made by the immediate family to donate organs and/or body tissue. That request was denied due to the possibility that Micotil was present in the victim.
ANALYSIS/SYNOPSIS
Cattle: The heifer in the squeeze chute was a purebred red angus, weighing approximately 800 to 840 lbs. The horned cow in the adjoining pen was a longhorn crossbreed weighing between 800-1000 lbs. The horns were approximately 12 inches in length and stuck straight out, unlike the side-to-side horns of most longhorn cattle. It could not be determined if only the force of impact knocked the victim down, or if a portion of the cow’s head and/or horns struck him and/or the syringe. Family members stated that there appeared to be impact marks of some type on the victim’s coveralls near the possible injection site.
Syringe/needle: The syringe in use that day was a 6 inch long, 12 cc Monoject 200™ with an 18 gauge, 1 ½” long needle (identical to that seen in photo #5). It could not be determined whether the protective cap was on when the victim left the “vet room” and/or when he was struck. Although intended for single use, it is common practice amongst cattlemen to use this type of needle for several injections. The investigator was not able to determine if the incident syringe/needle was new or had been used before. The victim was right handed and was believed to be carrying the syringe/needle in that hand, which was the side of his body that was impacted. It is believed, based on the weight of the heifer and the Micotil 300® dosing instructions that the syringe would have contained between 10-12 ccs. After the incident the syringe contained approximately 1.5 cc’s. It was not medically determined how much was injected into the victim. The needle, either during the impact from the cow or when the victim fell to the ground, stuck the victim in the right side groin area. Examination of the insulated coveralls by hospital personnel showed an area where it is believed the needle penetrated through the outside layer, which would indicate it was being carried in his hand. Family members stated that examination of the incident needle and the luer-tip of the syringe showed that both bad been drastically bent, presumably from the impact and accidental injection.
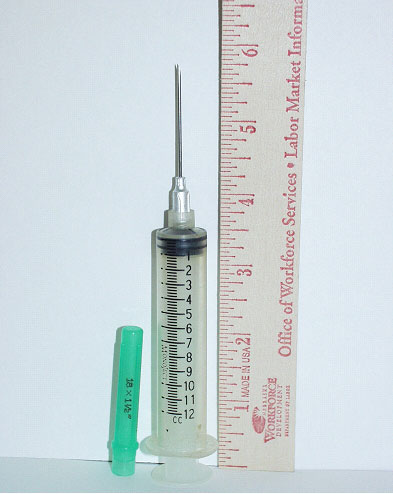 |
| Photo #5. The syringe in use that day was a 6 inch long (with protective cap installed) 12cc Monoject 200™ with an 18 gauge 1½ inch long needle. With the cap on the needle tip is approximately ¼ inch from the end. |
Note: The investigator did not have access to either the incident clothing or syringe.
Antibiotic: ²Micotil, which contains Tilmicosin phosphate, is used to control respiratory disease in cattle (bovine respiratory disease), more commonly called Dairy Calf Pneumonia (DCP), a very expensive and difficult-to-treat problem. It was designed to provide a single-injection therapy intended to reduce stress on the animal, thus requiring less labor since it is a low-volume dose used at a single injection site. It reaches effective concentration levels in lung tissue in two hours and maintains effective concentration levels throughout the respiratory tract for three to four days. It works with the animal’s own immune system to destroy pathogenic bacteria.
Micotil is an antibiotic that originally offered a lower cost per treatment than many other antibiotics for this indication available at the time. It was first introduced in Canada in 1990, then in the United States in 1992 and immediately gained wide acceptance. It is currently being marketed in several countries throughout the world.
A dosage of 1.5 mL per 100 lbs. of animal weight is recommended. It is to be injected subcutaneously (beneath the skin) in cattle. It can not be administered intravenously in cattle, as that proves fatal. The manufacturer states on all product literature that it is not to be used with automatically powered syringes, presumably due to its hazards to humans or possibly inefficiency to administer subcutaneous injections via this method. Most cattlemen use some form of disposable plastic syringe for injection.
Elanco is the only producer of Micotil. It is sold, through a distributor, only to licensed veterinarians. The victim had used Micotil® for several years. It could not be determined where or when he purchased the antibiotic in use during the incident.
CAUSE OF DEATH
According to the death certificate, the cause of death was: Respiratory failure as a consequence of cardiac arrest as a consequence of lethal injection.
RECOMMENDATIONS/DISCUSSION
Recommendation #1: Veterinarians and animal health distributors, prior to releasing Micotil, should require the purchaser to sign an information fact sheet that indicates Micotil can be fatal in humans and that there is no antidote for this medication, every time they purchase the product.
Discussion: Informal telephone and on-site verbal surveys with veterinarians were conducted throughout this investigation. They have shown that some veterinarians always warn their customers, either verbally or through manufacturer’s supplied literature, every time they purchase Micotil. Others have stated that if there has been a long-term relationship between them and the user, they do not always warn them of the possible dangers, assuming they remember.
Verbal surveys conducted with the customers indicated that most had originally been told of the dangers of Micotil, but had become complacent until this incident. Several admitted that although they purchased the medication, they were not the actual person that injected it, leaving that task to a hired employee. Most said that they had not discussed specific Micotil dangers with their employees, but had trained them on proper injection techniques.
Elanco does provide “prescription pads” specific to Micotil to all its customers (see photos 6 & 7). This sheet is intended for use by the issuing veterinarian or animal health distributor each and every time they distribute Micotil to a user. The front side contains general information about the user, injection dosage, suggested injection sites, comments by the issuing veterinarian and a line for the veterinarian’s signature. There is no signature line for the purchaser. A completed copy should be placed in the purchaser’s file, and a copy sent with the purchaser.
Suggestion: Add a signature line for the “purchaser”. Their signature will ensure that the information was presented to them and that they had opportunity to ask questions from the issuing veterinarian or animal health distributor.
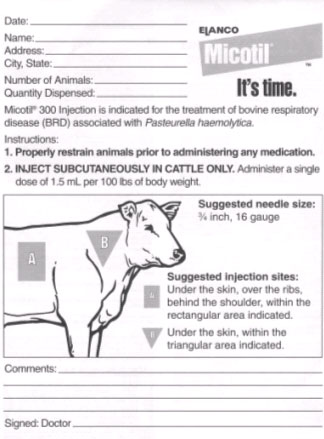 |
| Photo #6. Front of prescription pad. The shaded areas are light blue on original form. The actual paper size is 5 ½ inches wide by 8 ½ inches long. |
 |
| Photo #7. Back side of prescription pad. Note the Human Warnings are also in Spanish. |
Recommendation #2: Users of syringe-loaded medications should practice safe handling procedures during all phases of animal treatment.
Discussion: The victim had used this product many times before. Even though his cattle had the reputation of being extremely gentle and easy to work with, he still placed the heifer in the squeeze chute to medicate her, for both his and the animal’s safety.
Syringes should always be transported, whether full or empty, with the protective needle cap on. The investigator could not determine if the syringe was capped or not. The hard plastic needle cap, although extremely rigid and generally tight fitting, may not have been able to withstand the force of a direct blow from the horned cow if it were to contact the needle directly, or from an individual falling directly on the needle tip. The tip of the needle, when fully capped on this model, is approximately ¼ inch from the protective cap end.
Recommendation #3: Veterinarians/Cattlemen, when practical, should consider using another less-hazardous antibiotic.
Discussion: According to the Federal Drug Administration’s (FDA) Center for Veterinary Medicine and their Office of New Animal Drug Evaluation (ONADE), Micotil is not the only FDA approved veterinary drug without an antidote, but there is no published list of those medications.
The FDA does not require an antidote for any new animal drug that is approved. In order to be approved, veterinary drugs must be safe for the animal, for humans who consume products from the animal, and for the environment. In addition, they must be effective for the animal. Also FDA regulations require that adequate directions can be prescribed for the safe administration of the product, i.e. the establishment of a veterinarian/client/patient relationship.
The end-user can purchase Micotil from either a state licensed/registered veterinarian or an animal health distributor. In either situation, the end-user must have a valid prescription from a veterinarian before obtaining the product.
While an antidote is one possible solution to an accidental poisoning, it is not necessary to make the administration of the product safe. The FDA ensures that products are labeled properly so accidental injection and the need for an antidote do not happen if the user reads and understands the label and adheres to its recommendations. Micotil does carry warning labeling on the source bottle and also a warning sheet inside the container’s box that states in part “Not for human use. Injection of this drug in humans may be fatal…”. However, the warning sheet does not warn that there is no antidote to this medication.
For most drug development companies, during the discovery and testing phases, drugs are selected that ideally have therapeutic/toxicity safety margins built into the molecule, so that when they’re developed the toxicology is at non-lethal therapeutic doses or concentrations that hopefully human or animals will never experience. Although there have been other fatal cases in the United States associated with Micotil, all but two have all been ruled as “suicides” by law enforcement personnel. The remaining two do not have an absolute explanation.
The minimum amount of this medication needed to cause a fatality when injected into a human is not known. Interviews conducted with both veterinarians and users indicated they believed any amount greater than 6 ccs could prove fatal, depending upon the route of exposure or injection, e.g. subcutaneous, intramuscular, intravenous, oral, etc. ¹This may be based on a case of unintentional human exposure that occurred several years ago in Nebraska . The subject, a 28-year-old male, using a 12-cc syringe with Micotil, was attempting to inject a steer but inadvertently injected less than half of the contents into his left forearm. He felt no ill effects until approximately five hours later when he developed severe chest pain and was transported to a nearby hospital where he was intubated. He was extubated approximately 10 hours after arrival and remained free of chest pains for the 3 days of hospitalization and was discharged.
Several veterinarians queried during the investigation indicated they personally did not want to use this product due to the possible fatal human consequences. They all indicated that needle sticks in their business is unfortunately all too common, and to use a substance that may have no treatment depending on the amount and route injected or ingested was not their choice. They felt that there were other drugs on the market that would produce the same results and were safer to work with. Many indicated that since this incident they have received numerous calls from not only their customers about Micotil, but also from concerned family members that were looking for alternative medications.
Cattlemen like the drug because of its lower per dose volume, cost per treatment and reaction time. The majority of the current Micotil users that the investigator spoke with stated they would probably continue to use it, but be a little more cautious. Those that had employees had already discussed proper injection procedures with them after becoming aware of this incident.
Recommendation #4: All companies/agencies responsible for the manufacture and/or approval of veterinary medicines and supplies should continue to devise new products that will reduce unintentional human exposure to accidental needle sticks/injections.
Discussion: Interviews conducted during the investigation showed that users of disposable plastic syringes received needle sticks from a variety of situations. The preferred combination of having both medication and receiving animal next to each other, thereby reducing user exposure time, very seldom happens in the rural environment.
Elanco has developed a plastic shield for the 250-ml Micotil bottle that provides more protection to the user’s hand holding the bottle when inserting the needle. At this time it is only available for 250-ml bottles.
They also developed an Injection Administration Kit for hand operated syringes that allows multiple dosing, thereby reducing the number of needle/skin exposures. A center spike attached to tubing is inserted into the bottle, which is hung above the user.
The victim in this incident had only a few feet to travel from his vet room to the squeeze chute, but other users tell of traveling many miles with the loaded syringes to treat cattle. Unfortunately the majority of the time they place these syringes in bib overall pockets, toss them on the dash of the pickup, lay them on the vehicle’s seat next to them or place them in horse and ATV saddle bags.
An attempt was made to locate/identify some form of carrying case for these needles that would encapsulate the entire syringe/needle during transport, but none were identified by the investigator at the time of this report.
Suggestion: A device as simple as, and similar to, a hard plastic eyeglass case that would hold these plastic syringes with capped needles would further separate the user from possible accidental injection or exposure.
REFERENCES
ACKNOWLEDGEMENTS
While conducting this investigation the Nebraska FACE program requested the contents be reviewed for both the incident scenario & product/policy for factual data accuracy prior to publication.
Though we are not able to acknowledge specific individuals for their input, we would like to recognize the following for their support of this investigation:
• Victim’s immediate family
• Federal Drug Administration, Center for Veterinary Medicine
• Elanco Animal Health
• The Kendall Company
• National Institute for Occupational Safety & Health, Division of Safety Research
Disclaimer and Reproduction Information: Information in NASD does not represent NIOSH policy. Information included in NASD appears by permission of the author and/or copyright holder. More