Anhydrous ammonia is an efficient and widely used source of nitrogen fertilizer. Anhydrous ammonia has several advantages, including its relatively easy application and ready availability.
 However, there are also disadvantages and potential dangers
involved in handling anhydrous ammonia. It must be stored
and handled under high pressure, requiring specially designed
and well-maintained equipment. In addition, to ensure their
safety, workers must be adequately educated about the procedures
and personal protective equipment required to safely handle
this product.
However, there are also disadvantages and potential dangers
involved in handling anhydrous ammonia. It must be stored
and handled under high pressure, requiring specially designed
and well-maintained equipment. In addition, to ensure their
safety, workers must be adequately educated about the procedures
and personal protective equipment required to safely handle
this product.
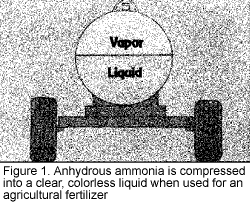
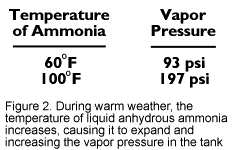
What is anhydrous ammonia, and why is it so risky to handle? It is a chemical made up of one part nitrogen and three parts hydrogen. The properties of this fertilizer make it one of the most potentially dangerous chemicals used in agriculture. Ammonia gas is colorless and has a sharp, penetrating odor. When used as an agricultural fertilizer, it is compressed into a liquid. In the liquid state, it is stored in specially designed tanks strong enough to withstand internal pressures of at least 2 0 pounds per square inch (psi) (Figure 1). As the outside temperature increases, the temperature of the liquid in the tank increases and the liquid expands, causing the vapor pressure in the tank to increase. For example, at 60 degrees F, the pressure is 93 psi and at 100 degrees F, the pressure is nearly 200 psi (Figure 2).
 If a hose ruptures or a valve is unintentionally opened, the
high pressure from the tank can cause ammonia to spray into
your eyes, face, and other parts of your body before you can
react. When pressure is released, liquid anhydrous ammonia
quickly converts to a gas.
If a hose ruptures or a valve is unintentionally opened, the
high pressure from the tank can cause ammonia to spray into
your eyes, face, and other parts of your body before you can
react. When pressure is released, liquid anhydrous ammonia
quickly converts to a gas.
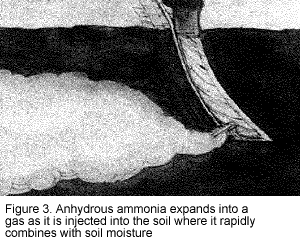
To protect yourself and other workers, you must be aware of the hazardous properties of anhydrous ammonia. The word "anhydrous" means without water. Consequently, when anhydrous ammonia comes in contact with any moisture, the water and ammonia rapidly combine. When injected into the soil, the liquid ammonia expands into a gas and is readily absorbed in the soil moisture (Figure 3). Similarly, in contact with your eyes, skin, or mucous membranes, ammonia will cause rapid dehydration and severe burns as it combines with the moisture of the body.
 Anhydrous ammonia boils at minus 28 degrees F. It must be
kept under pressure to be stored as a liquid above this temperature.
Thus, when liquid ammonia strikes the skin, it can instantly
freeze exposed tissue.
Anhydrous ammonia boils at minus 28 degrees F. It must be
kept under pressure to be stored as a liquid above this temperature.
Thus, when liquid ammonia strikes the skin, it can instantly
freeze exposed tissue.
Anhydrous ammonia is caustic and causes severe chemical burns. Body tissues that contain a high percentage of water, such as the eyes, skin, and respiratory tract, are very easily burned. Victims exposed to even small amounts of ammonia require immediate treatment with large quantities of water to minimize the damage.
Ammonia is also corrosive to certain metals, such as copper and zinc, and their alloys. Galvanized pipe must not be used for storing or applying ammonia because it contains zinc. Containers should be made of special high-strength steel or other approved material.
OPERATOR PROTECTION
 Because of the hazards associated with handling anhydrous ammonia,
operator protection must receive top priority at all times (Figure
4). Chemical-proof goggles, rubber gloves, and a heavy-duty
long-sleeved shirt are required for any operator handling ammonia.
In case of exposure, first aid must be administered immediately.
Plenty of water for flushing a person who has been accidentally
exposed to ammonia should be available at all times.
Because of the hazards associated with handling anhydrous ammonia,
operator protection must receive top priority at all times (Figure
4). Chemical-proof goggles, rubber gloves, and a heavy-duty
long-sleeved shirt are required for any operator handling ammonia.
In case of exposure, first aid must be administered immediately.
Plenty of water for flushing a person who has been accidentally
exposed to ammonia should be available at all times.
It is important to wear tight-fitting, chemical-proof goggles when handling ammonia. Regular glasses provide virtually no protection. Another option is to wear an approved full-face respirator that combines eye and lung protection. Never wear contact lenses when working with ammonia. Anhydrous ammonia can get under the lens and cause permanent eye damage before the lens can be removed.
Rubber gloves that are impervious to ammonia are required for handling anhydrous. The gloves should have an extended cuff that can be turned down at the elbow to prevent the chemical from running down your sleeve when your arms are raised. Gloves should have a fairly loose fit so they can be removed quickly in an emergency, but should fit tightly enough to provide adequate protection.
You can further protect your arms from ammonia spray by wearing coveralls or a heavy work shirt that covers the arms. Thin dress shirts or short sleeves do not provide protection.
 Regulations require that all anhydrous ammonia nurse tanks
and applicator tanks carry at least one five-gallon container
of clean water (Figure 5). This must be readily available
for flushing the eyes and skin in case of exposure. The water
should be changed daily to ensure a clean supply. It is also
recommended that a second five-gallon container of water be
kept on the tractor. This will provide the operator with another
source of water for first aid in case the operator is unable
to reach t e one on the nurse or applicator tank. Handlers
of ammonia should also carry an eight-ounce eye wash plastic
water bottle at all times in case an accidental exposure occurs.
Regulations require that all anhydrous ammonia nurse tanks
and applicator tanks carry at least one five-gallon container
of clean water (Figure 5). This must be readily available
for flushing the eyes and skin in case of exposure. The water
should be changed daily to ensure a clean supply. It is also
recommended that a second five-gallon container of water be
kept on the tractor. This will provide the operator with another
source of water for first aid in case the operator is unable
to reach t e one on the nurse or applicator tank. Handlers
of ammonia should also carry an eight-ounce eye wash plastic
water bottle at all times in case an accidental exposure occurs.
If you store bulk quantities of anhydrous ammonia, additional protective equipment is required. A rainsuit and two gas masks with ammonia canisters should be available for emergency work. The protection from a gas mask is limited, and a mask should only be used in low concentrations. If a serious leak occurs, call your local fire department for assistance. Firefighters have the proper training and equipment, including a self-contained breathing apparatus and protective suit, to deal with major leaks.
The operator's manual for anhydrous ammonia equipment should include instructions on proper procedures to follow when handling the product. Review this information before operating the equipment.
Note all decals on the equipment that identify valves and gauges involved in transferring ammonia. Decals should clearly identify the first aid water and other protective measures.
CONTAINER AND SYSTEM REQUIREMENTS
 The
specially fabricated and designed equipment for handling the
high pressures encountered with anhydrous ammonia should meet
the appropriate design guidelines provided by the American National
Standards Institute (ANSI). All parts and contact surfaces must
withstand a minimum working pressure of 250 psi. This includes
such things as pressure welds, safety valves, gauges, fittings,
hoses, and metering devices. Any repair or service work on tanks,
fittings, valves, and other components must be done by a qualified
technician with training and experience in repairing anhydrous
ammonia equipment.
The
specially fabricated and designed equipment for handling the
high pressures encountered with anhydrous ammonia should meet
the appropriate design guidelines provided by the American National
Standards Institute (ANSI). All parts and contact surfaces must
withstand a minimum working pressure of 250 psi. This includes
such things as pressure welds, safety valves, gauges, fittings,
hoses, and metering devices. Any repair or service work on tanks,
fittings, valves, and other components must be done by a qualified
technician with training and experience in repairing anhydrous
ammonia equipment.
All containers used for storing ammonia must be painted white or silver (Figure 6). Light colors reflect heat, helping to keep the temperature and pressure inside the tank at an acceptable level during warm weather.
Regularly scheduled maintenance is necessary to ensure that the tank and other components are ready for ammonia service. As nurse and applicator tanks become older, the hazards increase. Before using ammonia equipment, perform a walk-around inspection to locate any defects. Safety checklists are available from many anhydrous ammonia suppliers. Any parts found defective must be replaced or repaired. If this is not possible, the unit must be taken out of service.
 Proper care of the safety relief valve is an important part
of maintenance (Figure 7). This valve is designed to relieve
excess pressure from the vapor space of the tank at a predetermined
pressure setting. One organization recommends replacement
of safety relief valves every five years or if you discover
any leakage or other defects during your walk-around inspections.
Proper care of the safety relief valve is an important part
of maintenance (Figure 7). This valve is designed to relieve
excess pressure from the vapor space of the tank at a predetermined
pressure setting. One organization recommends replacement
of safety relief valves every five years or if you discover
any leakage or other defects during your walk-around inspections.
Ammonia hoses are considered the weakest link in the ammonia handling system (Figure 8). Hoses must be checked carefully before each use to make sure they can be safely used. You should replace hoses if you find bulges, cracks, cuts, soft spots, or blisters. Also, replace any hose that has begun to slip near the coupling. Use only hoses designed for anhydrous ammonia. Each hose should be marked with the words "anhydrous ammonia." In addition, the maximum working pressure, the manufacturer's name and trademark, and the year of manufacture and expiration date should be stamped on the hose.
Hoses are made with a variety of reinforcement materials and should be replaced accordingly. Hoses reinforced with rayon, nylon, and stainless steel should be replaced at two, four, and six years, respectively, from the date of manufacture, unless defects show up before this time.


Remember, safety while using ammonia is controlled by the judgement of the operator. If you find a defect, call your distributor for advice (Figure 9). Do not try to undertake a repair yourself. Again, only trained and qualified personnel should make repairs on anhydrous ammonia equipment.
AMMONIA TRANSFER
Review the procedures with any hired or family farm workers before allowing them to handle equipment to assure their safety. Make sure they fully understand the importance of protective equipment. Note that it is against federal law to hire any person below the age of 16 to transport, transfer, or apply anhydrous ammonia.
When filling a nurse or applicator tank, be thoroughly familiar with the equipment and procedures prior to transfer. Because most accidents occur when transferring ammonia, it is very important to wear your goggles and rubber gloves during these procedures. Make sure the five-gallon container is full of clean water, and that you have a small squirt bottle in your shirt pocket.
 Park the nurse tank on level ground, downwind from the filling
operation. Locate the nurse tank close to the source tank
to eliminate stretching or bending the hose. Avoid parking
too close to obstacles that would make evacuation difficult,
such as fences, buildings, or ditches. Block the wagon's wheels
and set the parking brake of the towing vehicle to prevent
movement of the nurse tank. A serious situation could develop
if the tank moves and a hose tears loose during the filling
operation.
Park the nurse tank on level ground, downwind from the filling
operation. Locate the nurse tank close to the source tank
to eliminate stretching or bending the hose. Avoid parking
too close to obstacles that would make evacuation difficult,
such as fences, buildings, or ditches. Block the wagon's wheels
and set the parking brake of the towing vehicle to prevent
movement of the nurse tank. A serious situation could develop
if the tank moves and a hose tears loose during the filling
operation.
Before connecting the hose, make sure the coupling and connections are free from dirt and other foreign material. Visually check to make sure that the threads are not damaged. This will reduce the chance of an ammonia leak under pressure.
Workers should carry the filler hose only by the valve body or coupling, not the valve wheel (Figure 10). This will reduce the chance of the valve wheel opening and spraying ammonia. Remember that the valve wheel and fitting are designed to be closed by hand pressure only. Do not use a wrench to close the valve wheel, since this can easily damage the fitting.
 If you are using a compressor to transfer ammonia, follow
recommended instructions in your operator's manual. Generally,
these include maintaining a vapor pressure of five to ten
pounds lower in the tank being filled to keep a forward flow.
If you are using a compressor to transfer ammonia, follow
recommended instructions in your operator's manual. Generally,
these include maintaining a vapor pressure of five to ten
pounds lower in the tank being filled to keep a forward flow.
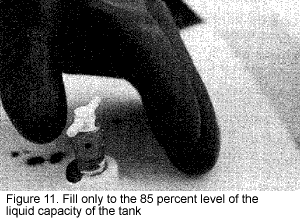
Do not overfill the nurse or applicator tank. Carefully monitor the liquid level by opening the 85 percent fill bleeder valve (Figure 11). A white fog will appear near the valve when it reaches this level. As a part of the normal loading procedure, check the liquid level float gauge accuracy by comparing it with the fixed liquid level gauge.
It is important to fill only to 85 percent or less of the total liquid capacity of the tank (Figure 12). Otherwise, as the outside temperature increases, the temperature of the liquid increases, causing the liquid to expand. This causes the vapor pressure in the tank to rise to a potentially dangerous level. If the tank is overfilled and no vapor space is available, the safety relief valve might fail, causing the tank to rupture or explode.
 After the filling operation is completed, secure the hose
in its storage position for transit. Take a final walk around
the nurse or applicator tank to confirm that all steps have
been taken.
After the filling operation is completed, secure the hose
in its storage position for transit. Take a final walk around
the nurse or applicator tank to confirm that all steps have
been taken.
A trailing nurse tank and tool bar are commonly used to apply anhydrous ammonia. Recommended safety precautions must also be used for this equipment. Provide a break-away coupler in the ammonia line that runs to the tool bar (Figure 13). Make sure this coupler is in good working order. This provides protection if the equipment separates accidentally. The valves in the coupler will stop the ammonia gas from escaping in both the upstream and downstream lines. Before reconnecting the coupler, remove the internal pressure from the lines both up and downstream of the coupler. This is done by opening the bleeder valves on each line. Failure to release this pressure could expose you to pressurized ammonia.
 Special precautions are necessary when removing dirt from
clogged applicator tubes. Because pressure can build up in
the tubes if they become clogged, a rush of anhydrous ammonia
can be expected when they are unclogged. Wear your goggles,
gloves, and a long-sleeved shirt while unclogging tubes. Position
yourself upwind from the clogged tube. You can use a long
piece of heavy-gauge wire to remove soil and other debris
from the tube.
Special precautions are necessary when removing dirt from
clogged applicator tubes. Because pressure can build up in
the tubes if they become clogged, a rush of anhydrous ammonia
can be expected when they are unclogged. Wear your goggles,
gloves, and a long-sleeved shirt while unclogging tubes. Position
yourself upwind from the clogged tube. You can use a long
piece of heavy-gauge wire to remove soil and other debris
from the tube.
FIRST AID


Even if small amounts of ammonia enter the eyes, flush them immediately with water for 15 minutes or more. Hold the eyelids open during irrigation to ensure water contact with all parts of the eye. This can be difficult and painful, but it is an important step to minimize the damage caused by ammonia. Immediate first aid is very important to avoid partial or total loss of vision. Again, consult a doctor after giving emergency first aid.
Ammonia vapors are easily detected because of their pungent odor, allowing them to be detected even in low concentrations. Inhalation of ammonia can irritate the respiratory tract and lungs. At high concentrations, ammonia that combines with moisture in the lungs may damage the lung lining. This can dramatically reduce the lungs' ability to transfer oxygen to the bloodstream.
If a person has inhaled ammonia, move the victim to a safe area. Exposures to low concentrations of ammonia for a short period of time may not require treatment. Exposure to higher concentrations may cause convulsive coughing and respiratory spasms. Provide cardiopulmonary resuscitation if the victim is not breathing, and summon emergency medical assistance.
If ammonia is swallowed, contact a doctor immediately. If the person is conscious, have him/her drink large amounts of water to dilute the chemical. Never give fluids to or induce vomiting in a victim who is in shock or unconscious. If vomiting occurs, keep the head lower than the hips to prevent vomitus from entering the lungs.
ROAD SAFETY
In many states, nurse tanks mounted for transport are considered "implements of husbandry" when used for agricultural purposes. They must conform to state regulations for travel on public roads. Nurse tanks must have the words "anhydrous ammonia" and "nonflammable" in large green lettering on both sides and on each end. All four sides of the tank must also have a placard displaying the "1005" identification number, and must have the words "inhalation hazard" on two sides. Applicator tanks must have markings identical to those on nurse tanks. These markings allow other motorists to easily identify an ammonia tank. Anhydrous ammonia tanks should also display a slow-moving vehicle sign clearly visible from the rear (Figure 16).
All anhydrous tank wagons must be securely attached to the towing vehicle by a drawbar, hitch pin and safety clip, and suitable safety chains. Be sure to check these parts to see that they are secure before each trip on the highway.
Anhydrous
ammonia wagons are designed to follow smoothly in the path
 of the towing vehicle. Tanks can readily overturn or collide
with another vehicle if the wagon swerves from side to side.
Make sure your tank wagon is hitched properly to prevent swaying.
Check to see that wheel lug nuts are tight and tires are in
good shape and properly inflated.
of the towing vehicle. Tanks can readily overturn or collide
with another vehicle if the wagon swerves from side to side.
Make sure your tank wagon is hitched properly to prevent swaying.
Check to see that wheel lug nuts are tight and tires are in
good shape and properly inflated.
When towing a nurse tank, drive at speeds of 30 mph or less. In Minnesota, anhydrous ammonia tanks and other implements of husbandry with a gross weight exceeding 6,000 pounds must not be towed faster than 25 mph, unless the wagon is equipped with brakes. The potential for a serious accident increases at higher speeds because the operator may lack sufficient braking capacity to safely control the wagon. Hauling more than one loaded nurse tank is a violation of the law in most states. Because arm implement tires are designed for low-speed travel, allow sufficient time to reach your destination.
In most states, the law has additional requirements if a nurse tank or applicator tank is towed between the hours of sunset and sunrise, and any other times when driver visibility is impaired. For example, in Minnesota, state law currently requires that anhydrous ammonia wagons be equipped with two red reflectors at the extreme left and right rear sides of the end of the wagon. Also, the widest portion of the towing vehicle/wagon combination must display a red or amber light at the extreme left end of the combination visible from the rear, and an amber or white light visible from the front. This means that if the wagon is wider than the towing vehicle, the wagon must be equipped with lights during low-light conditions.
OTHER USES FOR ANHYDROUS AMMONIA
Anhydrous ammonia is also used to add nonprotein nitrogen to corn silage. The ammonia is contained under pressure up to a cooling reactor in this application. Goggles, rubber gloves, and heavy-duty clothing, including long-sleeved shirts, should be worn during the process of connecting and disconnecting the ammonia hose and fitting.
SUMMARY
OTHER SOURCES OF ANHYDROUS AMMONIA SAFETY INFORMATION
ACKNOWLEDGMENTS
The authors wish to acknowledge technical assistance from William Belk, Loss Control Coordinator, Cenex Insurance Agency; Dr. Lynn Solem, St. Paul Ramsey Medical Center; and Craig Sallstrom, Executive Director, Minnesota Plant Food and Chemical Association. The authors express appreciation to Farmers Union Co-op, Hampton, MN; Glencoe Butter and Produce Association, Plato, MN; and Cannon Valley Co-op, Northfield, MN, for their assistance in providing material and services in obtaining photographs.
The information given in this publication is for educational purposes only. Reference to commercial products or trade names is made with the understanding that no discrimination is intended and no endorsement by the Minnesota Extension Service is implied.
Publication #: FO-2326-C
This document was produced by the Educational Development System, Minnesota Extension Service. This material is available in publication form, typeset, designed, and possibly containing artwork, tables and/or photos. For more information, contact MES, Distribution Center, 20 Coffey Hall, University of Minnesota, St. Paul, MN 55108-6069 (fax orders to 612/625-6281 or e-mail them to orders@dc.mes.umn.edu). Revised 1994.
John M. Shutske, Extension Agricultural Health and Safety Specialist. This publication is a revision of a previous one written by Robert A. Aherin and Lee Schultz. Copyright (c) 1994 Minnesota Extension Service, University of Minnesota. (612) 625-9733.
Disclaimer and Reproduction Information: Information in NASD does not represent NIOSH policy. Information included in NASD appears by permission of the author and/or copyright holder. More